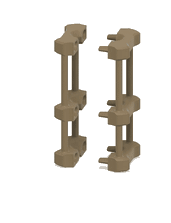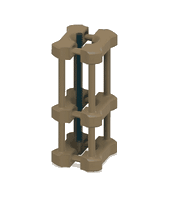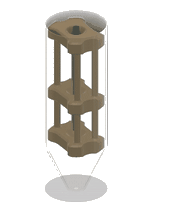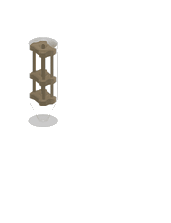Prepare all aqueous solutions using ultrapure water (prepared by purifying deionized water, to attain a sensitivity of 18 MΩ-cm at 25 °C). For non-polar preparations, use glass pipettes, adhere to proper safety precautions regarding volatile agents, and follow all waste disposal regulations when disposing of waste materials.
1.1 3D-Printed Microfluidics Device
1. 3D-printed microcapillary holder: SLA printed microcapillary holder, printed in Accura 60 material. Printed in high resolution (XY plane: +/- 0.005” for the first inch, plus +/- 0.002” for every inch thereafter. Z plane: +/- 0.010” for the first inch, plus +/- 0.002” for every inch thereafter) (see NOTE 1).
2. Glass microcapillary: 1 mm OD microcapillary with 10 um pulled tip.
3. Glass microcapillary scoring file: Blade shaped medium grit ruby degussit abrasive file.
4. Conical centrifuge tube: 1.7 mL conical microcentrifuge tube with 2 cm throat length and 9 mm ID.
1.2 Lipid-Oil Solution
1. Lipid-Oil Solution use in this demonstration was: 4.925 mM POPC, 4.925 mM DOPC, 0.15 mM fluorescently labeled PE (NBD-PE, N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)-1,2-Dihexadecanoyl-sn-Glycero-3-Phosphoethanolamine).
2. Prepare a 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) stock solutions by adding a non-specific amount of each powdered lipid species to separate massed glass vials.
3. Mass the vials to determine the amount of each lipid present within their respective vials.
4. Dissolve the powdered lipids within the vial in 5 mL of CHCl3 and recorded the measured concentrations of each solution using the observed mass of each lipid.
5. Use these solutions of known concentrations to prepare a singular 1:1 9.85 mM DOPC/POPC solution; however, do not adjust this solution to its final volume yet.
6. Add 0.15 mM of the desired fluorescently labeled lipid and subsequently bring the solution up to its final volume. At this point one should be left with a 10 mM 1:1 DOPC/POPC solution doped to 1.5 mol% with a fluorescently labeled lipid.
7. Aliquot 20 uL (for a 1 mM lipid-oil solution) or 10 uL (for a 0.5 mM lipid-oil solution) of this solution into brown HPLC glass vials.
8. Leave to desiccate inside a fume hood for minimum 24 hours, or desiccate under gas flow. (see NOTE 2 and NOTE 3).
1.3 Biomimetic Lumen Chemistries
Example RNA aptamer Broccoli transcription
1. The reaction was prepared as previously described, with the final concentration of all reagents: 4 mM each NTP, 40 mM tris pH 7.9, 42mM MgCl2, 100 mM KCl, 2 mM spermidine, 1 mM DTT, 2 μL 1 mM DFHBI-1T Broccoli aptamer ligand, 2 μL 5 μM Oligonucleotide Broccoli DNA template duplex, 2 μL 10X T7 RNA polymerase, 2 μL 10X Inorganic pyrophosphatase.
2. The Broccoli template used in this work was, the sense strand: d(TAA TAC GAC TCA CTA TAG GAG ACG GTC GGG TCC AGA TAT TCG TAT CTG TCG AGT AGA GTG TGG GCT C).
Example TX;TL of Green Fluorescent Protein (GFP).
1. The reaction was prepared as described before, using TxTl lysate of E. coli Rosetta DE3 strain and sonication protocol.
2. The final concentration in the reaction mixture was 500 mM HEPES pH 8, 15 mM ATP and GTP, 9 mM CTP and UTP, 2 mg/mL of E. coli tRNA mixture, 0.68 mM folinic acid, 3.3 mM nicotinamide adenine dinucleotide (NAD), 2.6 mM coenzyme-A (CoA), 15 mM spermidine, 40 mM sodium oxalate, 7.5 mM cAMP, 300 mM 3-PGA, 12mM Mg-glutamate, 140 mM K-glutamate, 1 mM DTT, 2 mM each of 20 amino acids, 10 nM GFP plasmid, RNAse inhibitor Murine 40U/ul 1x, 1 uM T7 RNA polymerase, cell free lysate 0.33x total reaction volume.
1.4 Liposome Preparation Buffer
1. To make liposome preparation buffer: mix 100mM HEPES, 900mM Glucose, pH 7.5.
2. Add about 100 mL water to a 1 L graduated cylinder or glass beaker.
3. Weigh 23.83 g HEPES and transfer to cylinder. Weigh 162.14 g Glucose and transfer to cylinder.
4. Add water to a volume of 900 mL.
5. Mix and adjust pH with HCl and NaOH respectively. Make up to 1 L with water.
6. Filter sterilize the buffer. Store at 4 °C.